 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Get your ComboX free sample to test now!
Get your ComboX free sample to test now!
 Time Limited Offer: Welcome Gift for New Customers !
Time Limited Offer: Welcome Gift for New Customers !  Shipping Price Reduction for EU Regions
Shipping Price Reduction for EU Regions
| Cat . Non | Espèces | Description du produit | Structure | Pureté | Caractéristique |
|---|---|---|---|---|---|
| CDA-HA2H6 | Human | Alexa Fluor™ 488-Labeled Human CD8 alpha Protein, His Tag |  |
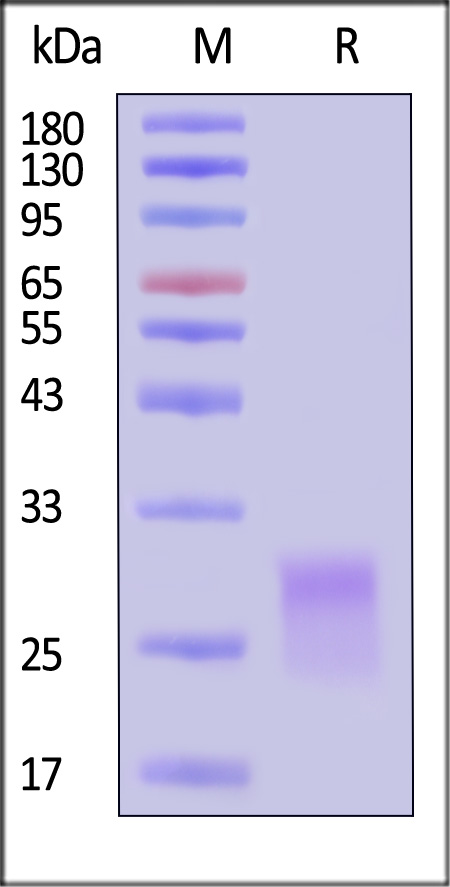
|
|
| CDA-C82E7 | Cynomolgus | Biotinylated Cynomolgus CD8 alpha / CD8A Protein, His,Avitag™ (MALS verified) |  |
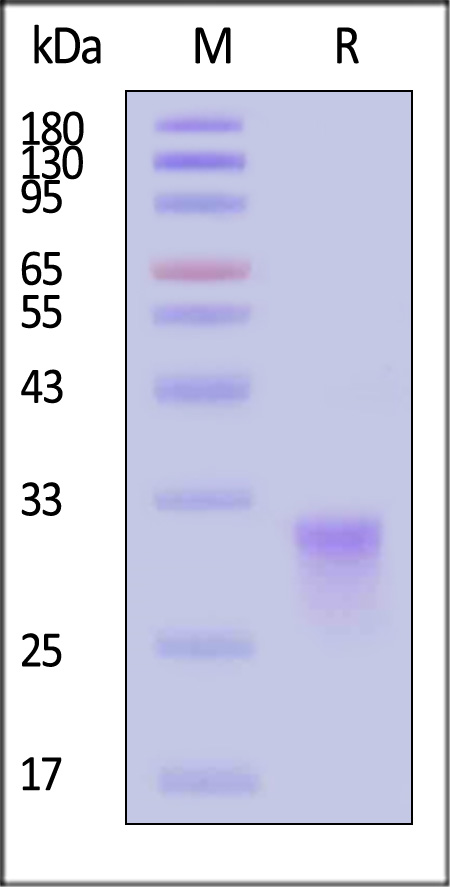
|
|
| CDA-C52H4 | Cynomolgus | Cynomolgus CD8 alpha / CD8A Protein, His Tag (MALS verified) |  |
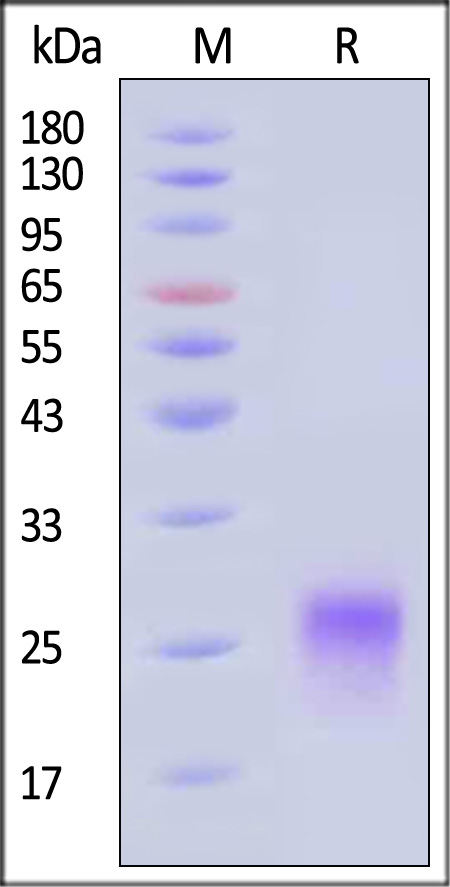
|
|
| CDA-H52H3 | Human | Human CD8 alpha / CD8A Protein, His Tag (MALS verified) |  |
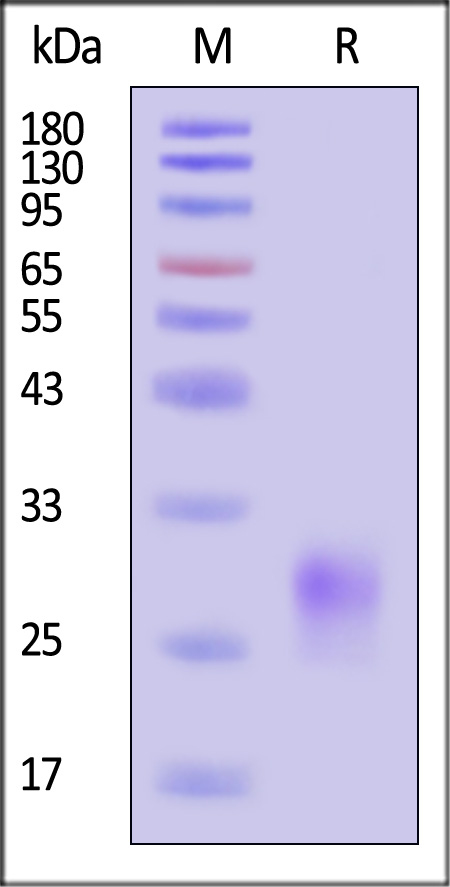
|
|
| CDA-H82E3 | Human | Biotinylated Human CD8 alpha / CD8A Protein, His,Avitag™ |  |
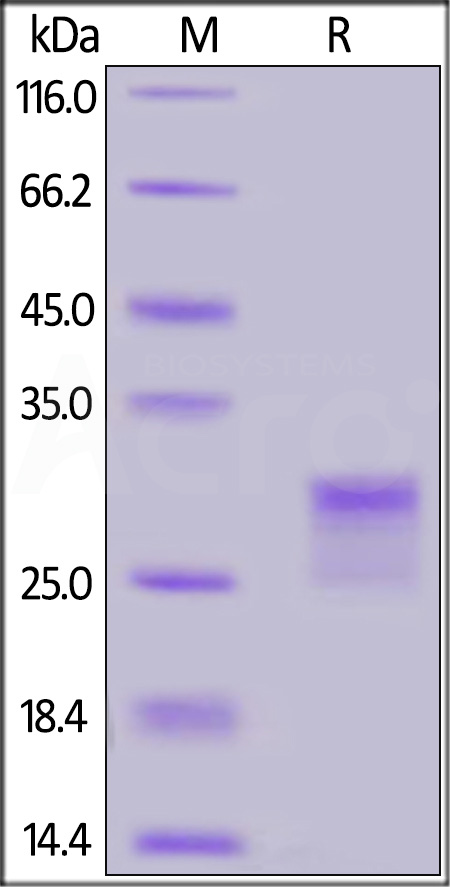
|
|
| CDA-M82E8 | Mouse | Biotinylated Mouse CD8 alpha / CD8A Protein, His,Avitag™ |  |
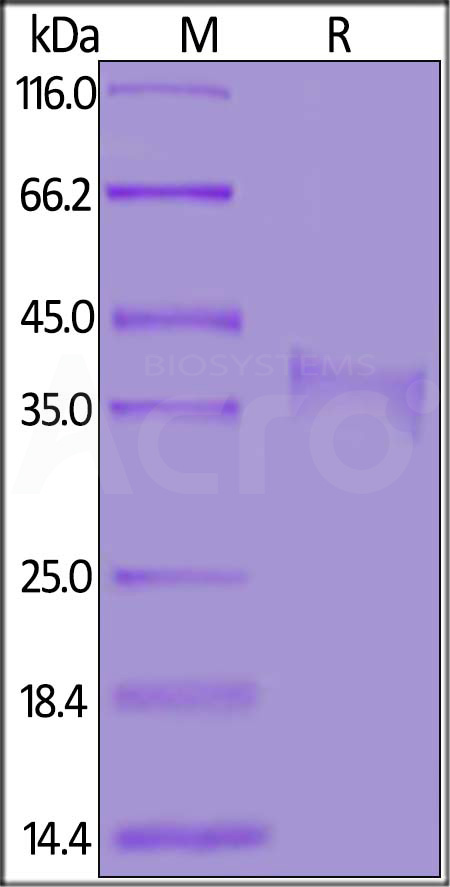
|
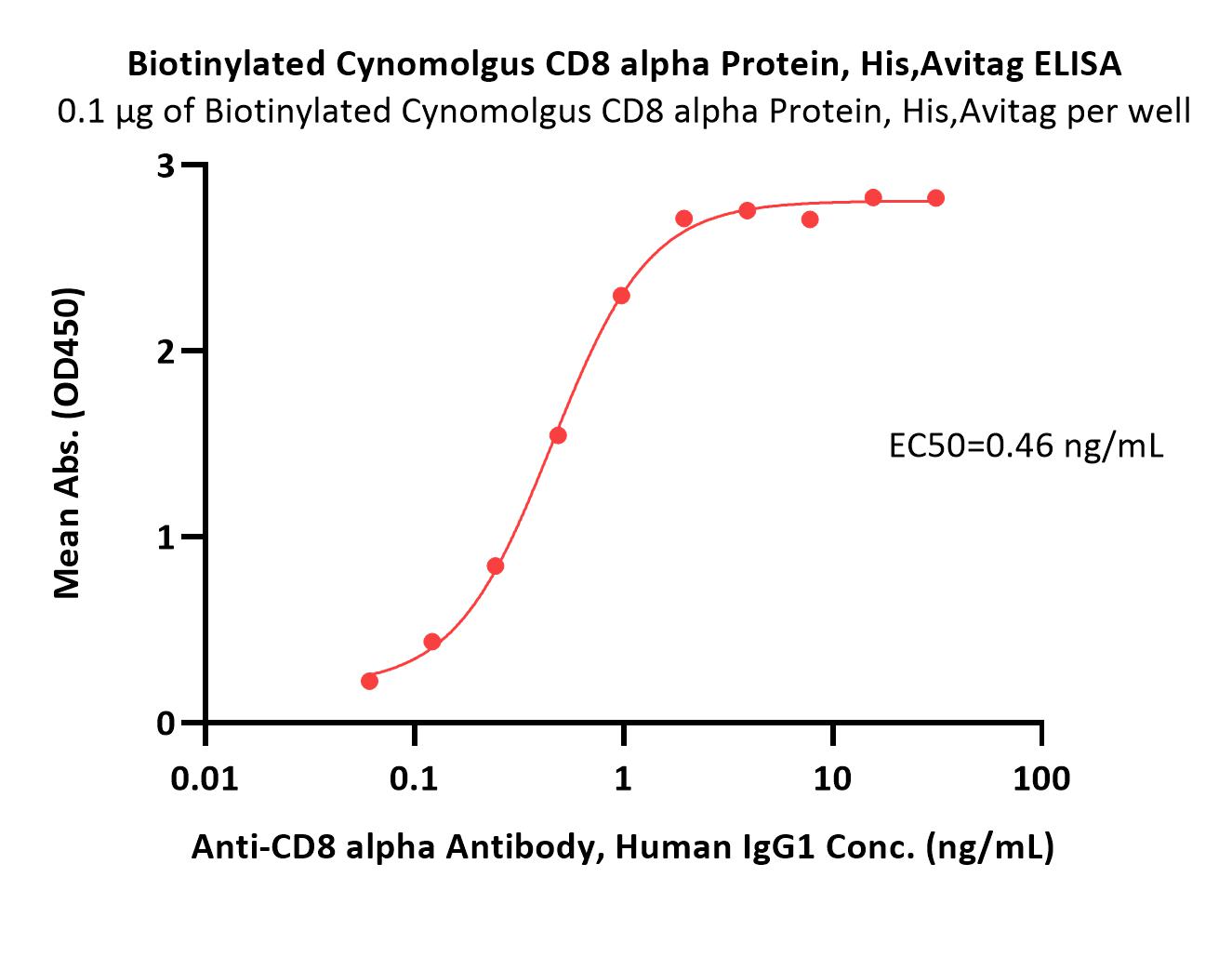
Immobilized Biotinylated Cynomolgus CD8 alpha Protein, His,Avitag (Cat. No. CDA-C82E7) at 1 μg/mL (100 μL/well) on streptavidin (Cat. No. STN-N5116) precoated (0.5 μg/well) plate can bind Anti-CD8 alpha Antibody, Human IgG1 with a linear range of 0.06-1 ng/mL (QC tested).
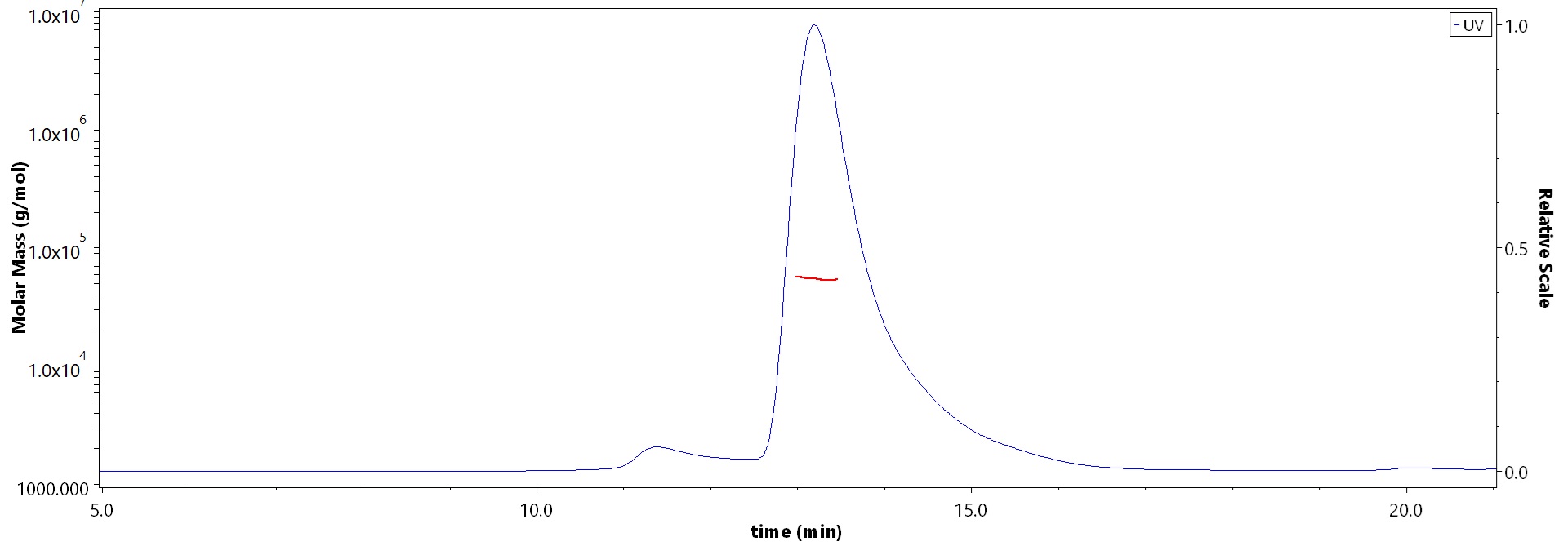
The purity of Biotinylated Cynomolgus CD8 alpha Protein, His,Avitag (Cat. No. CDA-C82E7) is more than 90% and the molecular weight of this protein is around 50-60 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| GRANITE | GRANITE-001; GRT-C901/GRT-R902 | Phase 3 Clinical | Bristol-Myers Squibb Company, Gritstone Bio Inc | Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Colonic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| 89Zr-Df-crefmirlimab | IAB-22-M2C | Phase 3 Clinical | Imaginab Inc | Solid tumours; Carcinoma, Merkel Cell; Carcinoma, Renal Cell; Multiple Sclerosis; Lymphoma; Leukoencephalopathy, Progressive Multifocal; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| RB-0003 | RB-0003; BNT-111 | Phase 2 Clinical | Biontech Se, Tron | Melanoma | Details |
| GEN-009 | GEN-009 | Phase 2 Clinical | Genocea Biosciences Inc | Skin Melanoma; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Transitional Cell; Carcinoma, Non-Small-Cell Lung | Details |
| VE-800 | VE-800 | Phase 2 Clinical | Vedanta Biosciences Inc | Esophageal Neoplasms; Stomach Neoplasms; Colorectal Neoplasms; Adenocarcinoma; Melanoma; Neoplasm Metastasis | Details |
| EBViNT Cell (Eutilex) | Phase 2 Clinical | Eutilex | Lymphoma, Extranodal NK-T-Cell | Details | |
| ZED88082A | ZED88082A | Phase 2 Clinical | Umcg The University Medical Center Groningen | Neoplasms; Neoplasm Metastasis | Details |
| NY-ESO-1-specific autologous CD8+ T-cell therapy (Fred Hutchinson Cancer Research Center) | Phase 1 Clinical | Fred Hutchinson Cancer Research Center, National Cancer Institute | Liposarcoma; Sarcoma, Synovial; Sarcoma | Details | |
| Tcrx T cell Therapy(Second Affiliated Hospital School Of Zhejiang University School Of Medicine) | Phase 1 Clinical | Second Affiliated Hospital School Of Zhejiang University School Of Medicine | Esophageal Neoplasms; Stomach Neoplasms | Details | |
| SCRI-E2CAR_EGFRtv1 | SCRI-E2CAR_EGFRtv1 | Phase 1 Clinical | Umoja BioPharma Inc | Osteosarcoma | Details |
| 68-Ga-NODAGA-SNA-006 | SNA-006; 68-Ga-NODAGA-SNA-006 | Phase 1 Clinical | Smartnuclide Biopharma | Solid tumours | Details |
| GRANITE | GRANITE-001; GRT-C901/GRT-R902 | Phase 3 Clinical | Bristol-Myers Squibb Company, Gritstone Bio Inc | Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Transitional Cell; Colonic Neoplasms; Colorectal Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| 89Zr-Df-crefmirlimab | IAB-22-M2C | Phase 3 Clinical | Imaginab Inc | Solid tumours; Carcinoma, Merkel Cell; Carcinoma, Renal Cell; Multiple Sclerosis; Lymphoma; Leukoencephalopathy, Progressive Multifocal; Melanoma; Carcinoma, Non-Small-Cell Lung | Details |
| RB-0003 | RB-0003; BNT-111 | Phase 2 Clinical | Biontech Se, Tron | Melanoma | Details |
| GEN-009 | GEN-009 | Phase 2 Clinical | Genocea Biosciences Inc | Skin Melanoma; Carcinoma, Renal Cell; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Transitional Cell; Carcinoma, Non-Small-Cell Lung | Details |
| VE-800 | VE-800 | Phase 2 Clinical | Vedanta Biosciences Inc | Esophageal Neoplasms; Stomach Neoplasms; Colorectal Neoplasms; Adenocarcinoma; Melanoma; Neoplasm Metastasis | Details |
| EBViNT Cell (Eutilex) | Phase 2 Clinical | Eutilex | Lymphoma, Extranodal NK-T-Cell | Details | |
| ZED88082A | ZED88082A | Phase 2 Clinical | Umcg The University Medical Center Groningen | Neoplasms; Neoplasm Metastasis | Details |
| NY-ESO-1-specific autologous CD8+ T-cell therapy (Fred Hutchinson Cancer Research Center) | Phase 1 Clinical | Fred Hutchinson Cancer Research Center, National Cancer Institute | Liposarcoma; Sarcoma, Synovial; Sarcoma | Details | |
| Tcrx T cell Therapy(Second Affiliated Hospital School Of Zhejiang University School Of Medicine) | Phase 1 Clinical | Second Affiliated Hospital School Of Zhejiang University School Of Medicine | Esophageal Neoplasms; Stomach Neoplasms | Details | |
| SCRI-E2CAR_EGFRtv1 | SCRI-E2CAR_EGFRtv1 | Phase 1 Clinical | Umoja BioPharma Inc | Osteosarcoma | Details |
| 68-Ga-NODAGA-SNA-006 | SNA-006; 68-Ga-NODAGA-SNA-006 | Phase 1 Clinical | Smartnuclide Biopharma | Solid tumours | Details |
This web search service is supported by Google Inc.
